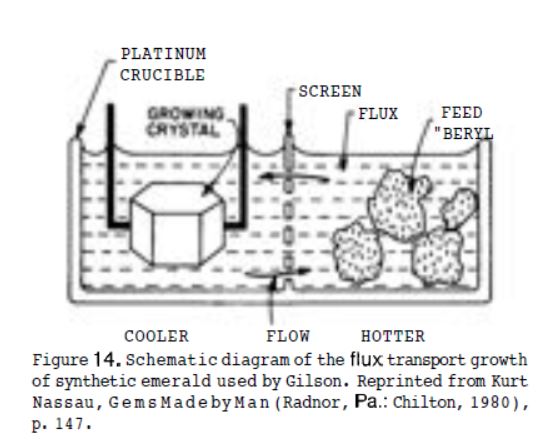
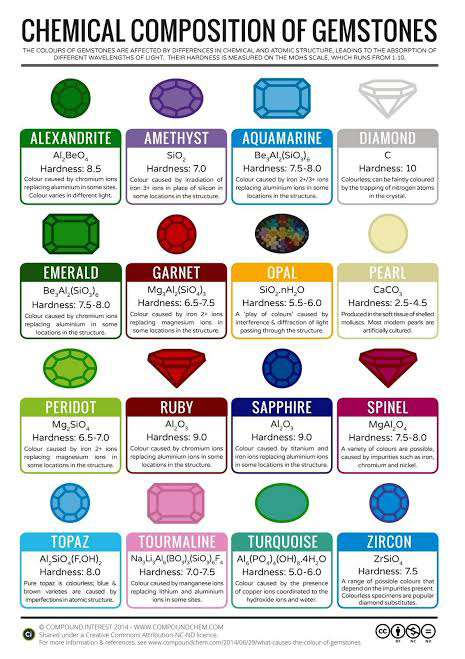
14 December 2019
History, nature, nhm, existing documentation, synthetic growth, my direction – dendrites? internship
bookmarks on crystal reading
Crystal & gem / written by Dr R.F.Symes and Dr R.R.Harding.
https://sciencing.com/create-synthetic-rubies-6788778.html
http://www.madehow.com/Volume-4/Synthetic-Ruby.html
Crystal growers make use of certain fundamental aspects of natural laws; these laws govern the states of matter and the interactions of atoms and molecules. O n e such law, known as the second law of thermodynamics, states that natural processes tend to run in a direction that produces the greatest entropy. Entropy is the degree of disorder, or randomness, of a system. The tendency of natural processes (that is, the direction in which things tend to go if left entirely to themselves) is to move in the direction of lowest energy. In other words, natural processes spontaneously go in a way that releases rather than absorbs energy. It turns out that energy is released when an atom or molecule attaches itself to an already-existing cluster of other atoms and molecules (a nucleus). T he major focus of crystal growers is to use this principle in a 277 272 GEMSTONES FROM THE LABORATORY controlled way.
https://designfactory.art.blog/wp-content/uploads/2020/01/26640-colorencyclopediaofgemstones-smallversion.pdf
https://www.alexandrite.net/chapters/chapter7/methods-of-producing-synthetic-alexandrite.html
https://www.alexandrite.net/chapters/chapter7/methods-of-producing-synthetic-alexandrite.html
https://www.alexandrite.net/chapters/chapter7/methods-of-producing-synthetic-alexandrite.html
Further Research: Synthetic Crystallography