22 November 2019
After finding a fern-like salt crystal on my paintbrush, I was curious about the colour, shape and exact explanation for the deposition of salt. Since the initial crystal formation was a bit flaky, I lost some of the fern branches, but with time, the white and blue crystals became harder and more solid.

I wanted to try creating sush crystals with other kinds of salts, to see if the colour, hardness and shape changed.
- The First experiment was with some leftover elderflower presse with a little white vinegar, to see if sugar crystals deposited on a wool broom suspended with brass wire.


After 48 hours 
After 90 hours
No crystallization after 90 hours
2. Next I left a paintbrush in White Salt + White Vinegar + Green ink solution


After 90 hours

After 102 hours
Slow crystallization along edge of bristles and metal strip
3. Wool broom on brass wire suspended in Himalayan pink salt + White vinegar mix


After 90 hours
Crystallization along threads of wool.
4. Wooden stirring Stick with a copper strip wound on it, dipped in White Salt + White vinegar mix




After 72 hours

After 90 hours
This arrangement gave me a growing blue crystal like the plastic cup accident. From my readings on crystallization, I concluded that the absorbent wood (and paintbrush bristles in the earlier case) allowed the salt vinegar mix to travel upward to the copper (or metal) portion, and at the point of intersection of metal and wood, created the first crystals of copper sulphate on account of corrosion of copper. These copper sulphate crystals might then, have acted as seed crystals for the further deposition of salt in the crystallization process. As opposed to submerged crystallization, this method caused a slow capillary absorption of solution in a single direction of motion, allowing for a slow deposition of fern like salt shapes, rather than adhering to the seed crystal from all directions.
5. Tissue paper stuck on wire mesh sink catcher suspended above the surface of Pink salt + White Vinegar mix


After 90 hours
Crystallization along the length of tissue paper. No further evaporation.
6. Large paintbrush dipped in Pink salt + White Vinegar mix


After 90 hours
Crystallization of pink salt along bristles, rust and crystallization on metal strip
7. Makeup Sponges in White Salt + White Salt + Glitter mix
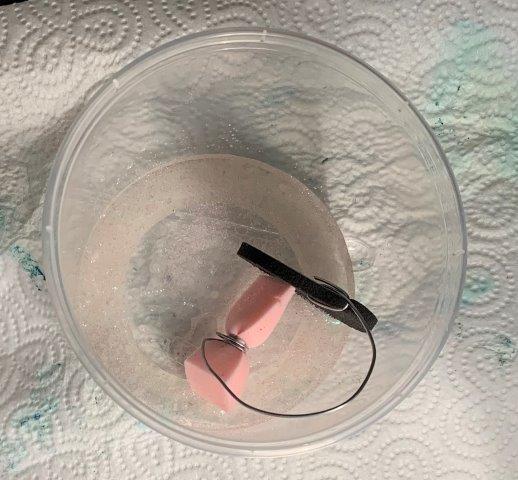

After 72 hours
No significant crystallization, not much absorption of solution on sponge
8. Wooden stick sipped in Epsom Salt (Bath Salt) + hot water mix
9. Bottle cleaner in Epsom salt solution + Green dye


After 60 hours
Crusts of epsom salt on the surface of solution, deposition at bottom of cup. No deposition on bottle cleaner.
10. Small paintbrush dipped in Epsom Salt + water (saturated) solution
11. Wooden stirring stick with steel strip wound on it dipped in Balsamic vinegar + Pink salt mix


After 60 hours
Some crystallization on wooden stick, none on steel strip
12. Wooden stirring stick + copper strip dipped in White salt + Balsamic vinegar + green dye (alcohol based) mix


After 60 hours
No travel of vinegar along stirring stick, no deposition of salt along stick or on copper strip
13. Holographic acrylic butterfly with glued seed crystals of white salt submerged in saturated white salt solution with water suspended with brass wire
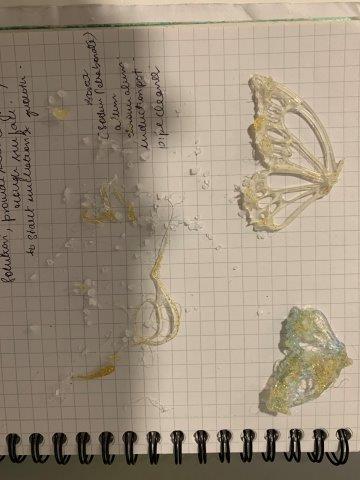


After 48 hours
Crystallization on seed crystals glued onto butterfly after 48 hours
14. Translucent plastic butterfly with glued seed crystals submerged in Saturated solution of white salt + green dye


After 48 hours
Crystals deposited at edge of cup competed with seed crystals on the plastic butterfly, thereby causing less deposition.
15. Copper sheet with punched holes stuffed with balsa strips and tissue paper placed above White Vinegar + White salt mix




After 8 hours 
After 30 hours
16. Copper punched circles + paper circles submerged in white salt + white vinegar mix

After 30 hours
No patina or crystallization after 30 hours – lack of oxygen molecules
Reflection
Relative overall success with copper strip wound along wooden stick – slow fern-like crystallization process. More experiments needed to test crystallization + patina effect in sheet of copper. To try – Copper Sulphate crystals with suspended (absorption and travel) arrangement. (Update: absorption did not result in any dendritic deposits with copper sulphate solution) To test – hardness of crystals – once solutions evaporate completely, to establish potential application options.




