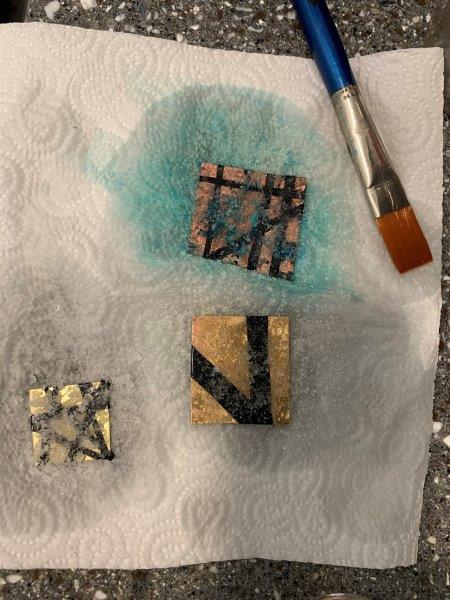05 November 2019
On my visit home during the break week, I found a brass cuff that I had cut and hammered from a thick brass sheet, a couple of years ago. With an easy access to ammonia (thanks to my pathologist mom), I went about trying to create a patina on the brass. (I had been thinking about patinas and rust on metals since the artefact project, where I wanted to create a rusted and worn effect on a watch movement)

Original hammered brass cuff 
Fuming process in a sealed plastic bag 
Patina gathered over 48 hours
- The first recipe was based on this video, basically requiring a ‘fuming’ of a white-vinegar dipped, and coarse rock-salt sprinkled metal piece in an air tight container with ammonia vapours. Since I did not have access to white vinegar, I used apple cider vinegar instead, hoping it would give similar results. The patina started coating my brass piece pretty quickly, and I let it stay in the plastic bag for about 2 days till I could see very little of the original ‘gold’ colour of the metal. Now that it was covered in black and hues of blue, I wasn’t sure of what to do with it, so I put it aside and bought some brass and copper washers to do some more smaller experiments.
2. This time I first dipped my brass and copper objects in vinegar, sprinkled with rock salt, and placed them within wads of tissue paper soaked in ammonia, based on this recipe. I realized that copper took on deep blue patinas almost immediately, while brass took time to develop the effect. I re-introduced my brass cuff to get a deeper patina through the wadding method.
3. I let the objects air dry after 24 hours (there was enough colour on them sooner, this time). Further, I applied clear nailpaint on some objects, to coat and seal the patina. This made the colours appear more vibrant, while giving the objects a little bit of gloss. While I like the brighter versions, I’m not sure I like the glossy effect, since the matte, rough look suits the process of aging of metal, aesthetically.
4. Back in London, I was inspired by Clare’s ‘uncovering’ and ‘recovering’ process of monoprinted ghost objects, as described by her during our Project launch presentation. I came home and started sanding the brass cuff to reveal some of its original ‘gold’ colour.
I quite liked the texture that the combination of hammering and patination brought to the cuff, revealing a contrast of the ‘old’ and the ‘new’.
5. Excited about this new technique, I tried the wadding process with copper washers in balsamic vinegar and fine table salt (for a lack of a fancier pantry). I avoided ammonia this time, on the basis of my reading of this article that explained the chemical processes behind the different colours achieved during the patination.
Equation 1: 4Cu + O2 → 2Cu2O [red to pink]
Equation 2: 2Cu2O + O2 → 4CuO [black]
Equation 3: Cu + S → CuS [black]
Equation 4: 2CuO + CO2 + H2O → Cu2CO3(OH)2 [“malachite,” dark green to blue]
Equation 5: 3CuO + 2CO2 + H2O → Cu3(CO3)2(OH)2 [“azurite,” blue to purple]
Equation 6: 4CuO + SO3 + 3H2O → Cu4SO4(OH)6 [“brochantite,” dark green to emerald]
https://www.worldcoppersmith.com/copper-patina-guide/
I thought that removing Equation 3 & 6 (basically removing the source of sulfur i.e. ammonia) would still allow the blue, red and black hues to appear in the presence of oxygen and water. Simultaneously, I put a similar set of vinegar dipped and salt sprinkled copper washers in a plastic bag with crushed, freshly boiled eggs. This was based on this recipe, which suggested the comparatively less smelly use of eggs as a source of sulfur to equations 3 & 6.
After about 24 hours, the washers wadded without being fumed with egg sulfur showed very little blue patination, and there was no black residue at all. Whereas, the washers exposed to crushed eggs showed not only more blue, but also had a darker, black and brown residue.
6. I felt that I needed to try white vinegar and coarse salt for the next experiment, since the rock salt would product bigger patterns of blue, and I wanted to see what white vinegar could do that balsamic and apple cider couldn’t.
Within only 3-4 hours, the white vinegar and coarse salt mixture (without eggs) gave a more dramatic patina effect than any of the previous experiments. Here, I found a yellow-orange colour added to the hues of the patina. The blues and blacks were more pronounced as well.

7. Next, I tried to mask certain areas of squares cut out of copper and brass sheet, to see if the patina would escape under the electrical tape, or if I could achieve the ‘old’ and ‘new’ contrast from my sanded brass cuff. I dipped my pieces in a mix of balsamic and white vinegar, and sprinkled with a mix of coarse and fine table salt. After about 4 hours of having left them wadded in the vinegar and salt mix, I observed very little patina effect on both the brass and the copper. I then added crushed eggs to see if any black residues would appear. I also refreshed the salt and vinegar on the pieces to intensify any patina effect.
Masked pieces: top-copper, bottom two-brass; after 4 hours

After about 24 hours – (20 hours in egg vapour)
Having removed the electrical tape, I noticed different effects in the 3 pieces. one of the brass pieces (left), bought from a jewellery supplies store, showed blue-green salt crust, and a clear gold in areas masked with tape. The other brass piece (middle) bought from a hardware store in India, showed slight pink patination around its edges, with brown smoke-effect patina in areas where the vinegar reacted with the glue from the tape. The copper piece showed thick deposits of bright blue salt and patina underneath, leaving clear trails under the masked areas. I’m not sure whether I’d like to sand these pieced down yet, or let them sit with their salt crusts…
8. For the next experiment, I’m trying to get sulfur fumes from heated garlic paste, having discovered that garlic is another organic source of sulfur (also trying to put off bringing ammonia into my room for as long as I can). With white vinegar and rock salt dipped copper and brass washers, this time, I m trying to speed up the patina process by warming apparatus over a double boiler.

After 2 hours – exposed to heated garlic vapours and sitting in lukewarm water. Top 2 washers – brass, Bottom 4 – copper.

After 12 hours in garlic vapour (8 hours at normal temperature)

Reflection:
These experiments have made me think about concepts of wear and aging in functional materials and objects, and have made me value the beauty in naturally worn out things (as opposed to or in combination with artificial distressing and wear?) I’m also interested in the contrasting textures created when ‘protecting’ certain parts of an object from the corrosion process. I’m not sure where these experiments sit within my Investigation for project 4, but I’m enjoying the material exploration nevertheless.